Birth defects related to chromosomal abnormalities often stem from exposure to chemicals early in the mother’s life. But determining which chemicals are at fault poses a serious challenge — akin to solving a hit-and-run case, decades after the fact. Two researchers in the UC Davis College of Biological Sciences are developing a method that could identify harmful chemicals far more quickly, with the help of red- and green-glowing zebrafish.
Their work could benefit millions of people in California’s Central Valley, who are at elevated risk for exposure to pesticides because they live or work near agricultural production sites. Pesticide exposure can cause both acute and long-term health problems in humans, including harm to the reproductive system. This harm often occurs because chemicals interfere with sensitive stages of fetal development, during which the cells that will one day produce sperm or oocytes are forming.
Finding generational effects
“You won’t see the effect until those children grow up and try to have children of their own,” said Sean Burgess, a professor in the Department of Molecular and Cellular Biology. At that point, women may experience infertility or repeated miscarriage; the children they bear may be at increased risk for Down syndrome or other serious conditions that are caused by having extra copies of chromosomes.
Burgess is working with Bruce Draper, a professor in the same department, to develop a technique that could greatly accelerate the screening of chemicals and more rapidly identify those with long-term reproductive effects. The gap between the chemical “hit-and-run” and reproductive consequences is often decades, said Burgess: “We’re shrinking that time down to basically weeks.”
Standard testing is slow and expensive, because it relies on mice, which must be individually dissected and examined by technicians to see the effects of chemicals on reproductive tissues. Burgess and Draper plan to circumvent this cumbersome process by using a newly developed strain of zebrafish (Danio rerio). This freshwater fish species, native to South Asia, is popular in home aquariums. It is also frequently used as a model organism to study the early stages of human development.
“Seventy percent of the genes in zebrafish have human counterparts, called orthologs,” said Draper. And if you look at the genes involved in oogenesis — the production of female oocytes, or egg cells — the percentage is even higher.
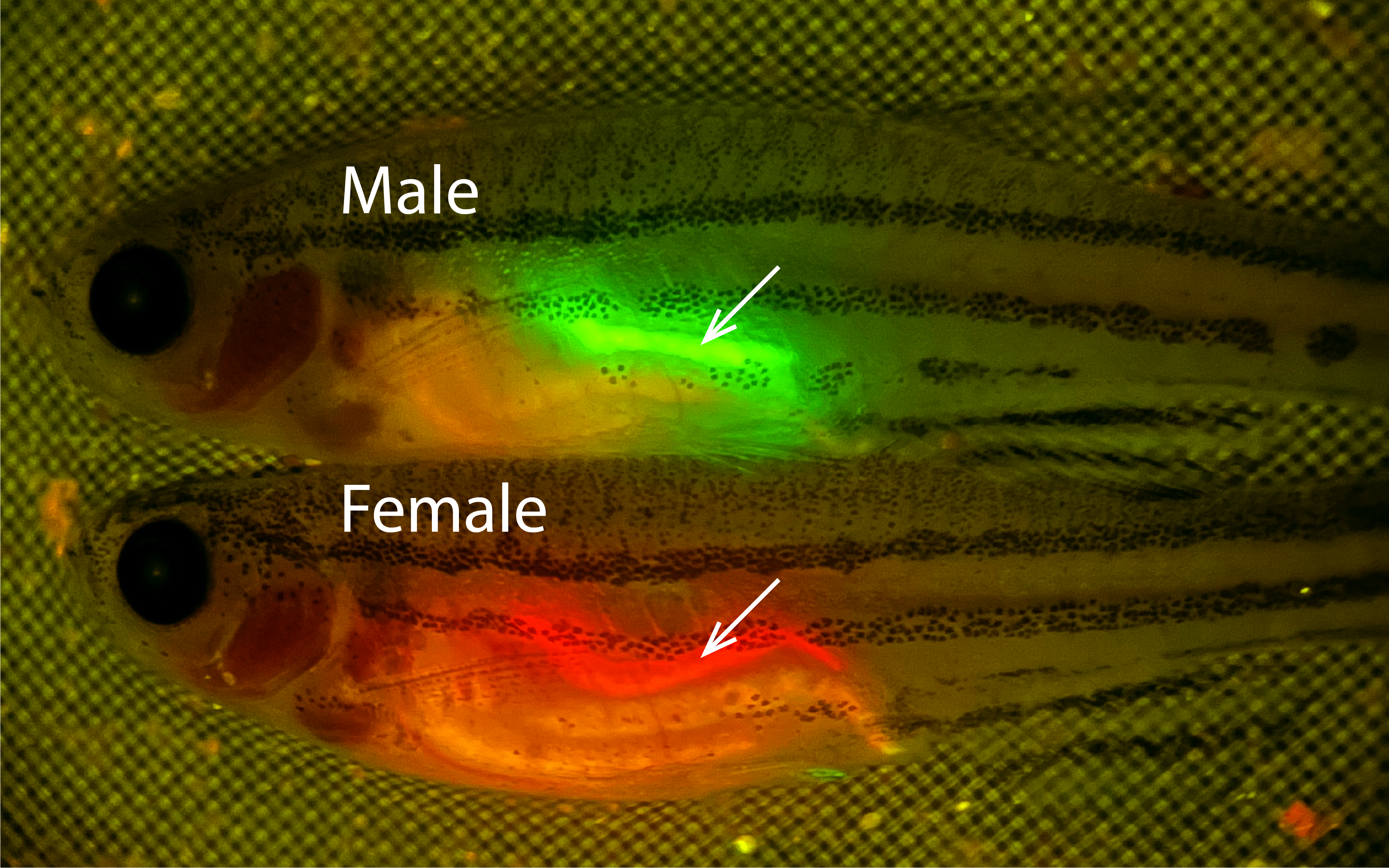
Zebrafish are well-suited for studying the reproductive effects of chemicals because unlike mammals, their sex is not determined by special X or Y chromosomes. Instead, it is determined in part by environmental cues. In captivity, roughly half of the fish develop into females. But if fish larvae are exposed to chemicals that disrupt oogenesis, then a higher percentage of them will develop as males. This means that scientists can screen a chemical for reproductive toxicity by exposing a few dozen zebrafish larvae to it — then waiting several weeks to see if their sex ratio is skewed toward males. Draper and Burgess are developing a strategy to do this — using genetically modified zebrafish that display their sex prominently, through color coding.
These fish, developed by members of Draper’s lab, carry three genetic changes. First, their Sertoli cells, found only in the male gonad, produce green fluorescent protein. Second, their oocytes (or immature eggs), found only in the female gonad, produce red fluorescent protein. And finally, the fish manufacture less of their natural pigment — making their bodies more transparent, so the red or green colors of their gonads show more clearly.
Zebrafish are easier and less expensive to care for than rodents. Burgess and Draper expect to raise 80 fish larvae in each tank, exposing the animals in each one to a selected chemical between 10 and 20 days post-fertilization. One would ordinarily have to wait until 90 days post-fertilization to distinguish male and female zebrafish visually. But the fishes’ color-coded gonads should allow them to do this at 40 days.
“We should be able to determine the sex of a cohort of 80 animals, almost simultaneously, just by taking a picture,” Draper said. Seeing an unusually high percentage of males or females, or seeing intersex animals with gonads shining both red and green, would indicate that the chemical is toxic to the reproductive system.
Glonad assay
In April this year, the UC Davis Environmental Health Sciences Center’s Pilot Projects Program awarded funding for Burgess and Draper to develop their “glonad” assay for toxicity screening. The two of them hope that later this year they can begin using it in a pilot experiment to screen nine of the most commonly used pesticides in California for reproductive effects.
That initial test could eventually pave the way for broader use of the glonad assay. Ninety pesticides are currently known to the state of California to cause birth defects or reproductive harm. But these toxicities could potentially be linked to a broader range of pesticides and other chemicals, such as bisphenols, which are used in manufacturing some plastics.
The real power of the glonad assay is that it could be scaled up to test many more chemicals beyond those that are currently possible. And each of those chemicals could be tested in large numbers of fish — allowing detection of even rare reproductive effects.
“It’s way more efficient than anything else out there right now,” said Draper. “We have high expectations that this is going to work.”
Media Resources
Douglas Fox is a freelance science writer based in the Bay Area.
Glonad: A Novel Assay for Reproductive Toxicants Using Zebrafish (Environmental Health Sciences Center)
