Quick Summary
- UC Health supports giving the vaccine to this age group
- UC Davis Health experts offer insights in Q&A and video
- Vaccinations due to be available Nov. 9 at UC Davis Health
University of California Health issued a statement today (Nov. 4) in support of the Centers for Disease Control and Prevention’s recommendation of the Pfizer-BioNTech COVID-19 vaccine for children ages 5 to 11.
VACCINE AT UC DAVIS HEALTH
- COVID-19 vaccinations for ages 5-11 will be available starting Tuesday (Nov. 9) at UC Davis Health. Appointments are available now in the state’s My Turn system.
- More information is available in this UC Davis Health post: How to Schedule Your COVID-19 Vaccine.
“The thorough review of the large amount of study data through the CDC process and by the Food and Drug Administration in its emergency use authorization shows the vaccine to be safe and effective for children,” UC Health officials said. “UCH encourages parents and guardians to have their eligible children vaccinated and to talk with a pediatrician if they have questions.”
Read the complete statement here. The statement carries the signatures of numerous experts from around the UC system, including UC Davis’ Dean Blumberg, chief of pediatric infectious diseases at UC Davis Children’s Hospital.
Blumberg appears in the video above, answering questions about vaccinating ages 5-11, and joins with Lorena Garcia, professor of epidemiology at the UC Davis School of Medicine, in answering the questions below about COVID-19 in this age group and about the vaccine for these children.
The CDC’s Nov. 2 recommendation followed by four days the decision by the Food and Drug Administration, or FDA, to grant emergency use authorization of the Pfizer-BioNTech vaccine for ages 5-11. California stands ready to make COVID-19 vaccination mandatory for in-person school attendance from kindergarten to 12th grade; this will take effect for each age group only after the FDA grants full approval of a vaccine or vaccines for that group. Full approval can lag behind emergency use authorization by several months.
As the vaccine rolls out for the 28 million U.S. children ages 5-11, here’s a look at the effectiveness of the vaccine: CDC estimates for August indicate that people who were unvaccinated were six times more likely to test positive for COVID-19 and more than 11 times more likely to die from it.
Q&A with our experts
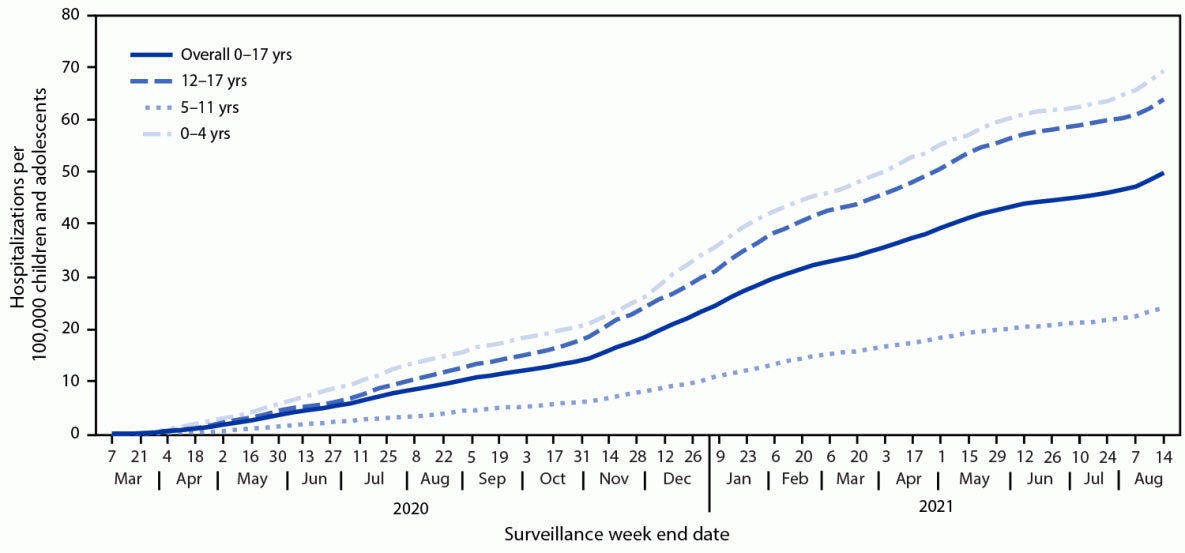
Lorena Garcia: To date, 1.9 million COVID-19 cases have been reported in children 5-11 years old (roughly 7% of that population). During the large wave last winter, children in this range had a lower rate of infection compared to adults; however, in the recent wave starting last July the rate increased and has remained closer to the rate for young adults. Hospitalizations in this age range have been the lowest of any age group but have also increased over time (see chart below), reaching 24 per 100,000 in August of 2021. One-hundred-sixty deaths have been reported in this age group to date, a rate of 0.008%. For comparison, 112 children ages 5-17 died due to influenza in the 2019-20 flu season. That number was slightly higher (156) in the 2018-19 flu season.
This graph is from the CDC's Morbidity and Mortality Weekly Report, Sept. 10, showing COVID-19-associated cumulative hospitalizations per 100,000 children and adolescents by age group in 14 states, March 1, 2020-Aug. 14, 2021.
Are there factors that increase susceptibility and the risk of hospitalization and death for children in this age range?
Dean Blumberg: Children with underlying medical conditions are at increased risk for more serious disease. This can include heart, lung or kidney diseases, weakened immune systems, or chronic illnesses such as obesity or asthma. In fact, two-thirds of children hospitalized with COVID-19 have at least one underlying condition. And children with obesity are 30% more likely to be hospitalized with COVID-19 compared to children without obesity.

Lorena Garcia: Socioeconomic factors including poverty, unequal access to health care, poor environmental conditions and educational inequities increase the risk of infection, severe illness and death in children in this age range. These often translate directly into a disparate impact on minority communities.
When normalized for population distribution, Black, Hispanic and American Indian and Alaska Native children have the highest rate of hospitalization and death — two to four times those observed for white and Asian children. Children of Asian descent had the lowest rate of infection, hospitalization and death.
A key intermediary for the disparate impact in these communities may be the (two to three times) higher rates of existing medical conditions described above by Dr. Blumberg. Differences in vaccination rates also contribute to the trends, with Asian children having the highest vaccination rates and Black children having the lowest.

Dean Blumberg: Multisystem inflammatory syndrome in children (MIS-C) occurs two to four weeks following acute COVID-19 infection and involves inflammation of several different organ systems. It may involve the heart, lungs, kidneys, brain, skin, eyes or gastrointestinal organs. MIS-C can be serious, even deadly (1%-2% die), with the vast majority of children hospitalized and more than half admitted to intensive care. Fortunately, most children who were diagnosed with this condition have gotten better with specific medical care. This occurs in approximately one out of every 3,000 children (up to 21 years of age) who get COVID-19. The most common age for MIS-C is 5-11 years of age. Racial and ethnic minorities are at increased risk for MIS-C, with over 60% of cases occurring in Hispanic/Latino or Black non-Hispanic children.
Dean Blumberg: Long COVID occurs when symptoms linger four or more weeks after an acute infection. There are a wide range of health problems associated with long COVID including headache, “fuzzy thinking,” fatigue, sleep difficulties, headaches, loss of taste and smell. It occurs in children but appears to occur about 50% less commonly compared to adults. A recent study found 7%-8% of children with COVID-19 reported continued symptoms more than 12 weeks after diagnosis. The impact on quality of life during this period may be significant with limitations of physical activity, feeling distressed about symptoms and mental health challenges. These may all result in decreased school attendance and participation.
Dean Blumberg: Some studies suggested that infection and transmission rates were lower in children compared to adults, but others suggest they are similar. Several factors including variants and vaccines may explain the different results. What we know with more clarity is that vaccination reduces the risk of infection, which in turn decreases the risk of transmission to others, including family members.

Lorena Garcia: The Centers for Disease Control and Prevention has provided guidance based on current scientific evidence and lessons learned from schools. The evidence shows that implementing layered prevention strategies can reduce the spread of COVID-19 to levels below community settings. These include wearing masks, washing hands, physical distancing, cleaning and disinfecting, ventilation, as well as testing, tracing and quarantining programs.
Dean Blumberg: The dose of the Pfizer/BioNTech vaccine is one-third the dose compared to older children and adults. It is not uncommon for pediatric vaccine doses to be lower than doses for older people since the immune response is robust in younger individuals?
The mRNA used in the vaccine is the same, but the use of buffering agents differ slightly to help maintain the pH and stability for extended storage periods.
Dean Blumberg: The pivotal study evaluated by the FDA for the emergency use authorization comprised 2,268 children 5-11 years of age. That sample size is similar to the study used for 12-15-year-olds. In the study, 1,518 children received two doses of vaccine 21 days apart, while 750 children received a placebo. There were three symptomatic COVID-19 cases in the vaccine group and 16 in the placebo group, resulting in 91% effectiveness.
The antibody responses after vaccination were the same compared to older children and young adults. The side effects after vaccination were also similar, although the younger children tended to have milder side effects. For example, pain at the injection site was twice as common after vaccination compared to the placebo, but severe pain was uncommon.
Dean Blumberg: Anyone with a severe allergic reaction to a previous dose of COVID-19 vaccine or any of the vaccine components should not be vaccinated. Some Scandinavian countries suspended Moderna vaccine use in children and young adults (under 30 years of age) due to concerns related to myocarditis or heart inflammation that can occur, rarely, after vaccination. The CDC and others have studied this in the U.S. and concluded that it is far safer for children to be vaccinated compared to being vulnerable to COVID-19 infection which may also cause myocarditis.
Lorena Garcia: Vaccines have been one of the most effective tools in reducing the risk of vaccine-preventable childhood diseases. Requiring a vaccine for students is not new. With the addition of COVID-19, California now requires 10 immunizations. The Pfizer/BioNTech vaccine uses a new approach called mRNA but offers a similar rate of effectiveness as the polio (99%), measles (97%) chickenpox (90%) and mumps (88%) vaccines. Each disease and related vaccine has its own risk/benefit profile. In terms of transmission, COVID-19 is less transmissible (three to seven transmissions per infection) than measles (12-18 transmissions) and chickenpox (10 transmissions), but similar to polio (four to six transmissions). The case mortality rate for COVID-19 (0.008%) is less than that for measles (0.1%) and polio (0.05%-0.025%) but higher than chickenpox (0.003%).
Lorena Garcia: As described above, the health and economic toll can have a larger effect in under resourced communities. In order to help mitigate the disparities, we need to ensure proportionate access to care and the vaccine, flexibility for time off to receive the vaccine and replacement of wages when sick or caring for a family member that is sick. For immigrants, we also need to address language barriers and fear of deportation. Community centered mitigation policies should include widely accessible integrated health care delivery, transportation to medical appointments, language services, community health workers, long-term community investment (e.g., affordable housing, jobs), and systematic collection of data, including race and ethnicity and the social determinants of health such as housing, food, transportation, utilities, child care, employment, education and finances. We also need mechanisms and community partnerships to deliver scientifically sound information regarding vaccines and diseases.
Media Resources
AJ Cheline, director of marketing and communications, and Neelanjana Gautam, communications specialist, Office of Research, contributed to this report.
Dateline Staff: Dave Jones, editor, 530-752-6556, dateline@ucdavis.edu; Cody Kitaura, News and Media Relations specialist, 530-752-1932, kitaura@ucdavis.edu.