A key phase in the repair process of damaged human DNA has been observed and visually recorded by a team of researchers at the University of California, Davis. The recordings provide new information about the role played by a protein known as Rad51, which is linked to breast cancer, in this complex and critical process.
The breakthrough comes a decade after Stephen Kowalczykowski, a distinguished professor of microbiology and the study’s principal investigator, and Ron Baskin, professor emeritus of molecular and cellular biology, first began developing methods of labeling molecules with fluorescent markers and observing them at work using optical trapping of individual DNA molecules and advanced microscopy techniques. In 2006, the researchers recorded a portion of the bacterial DNA repair process, a system considerably less complex than its human counterpart. The new study was published in the Proceedings of the National Academy of Sciences on Jan. 13.
To view video on this page, get the Adobe Flash Player and enable JavaScript.
Human DNA Repair Process
Flash video, (33 sec)
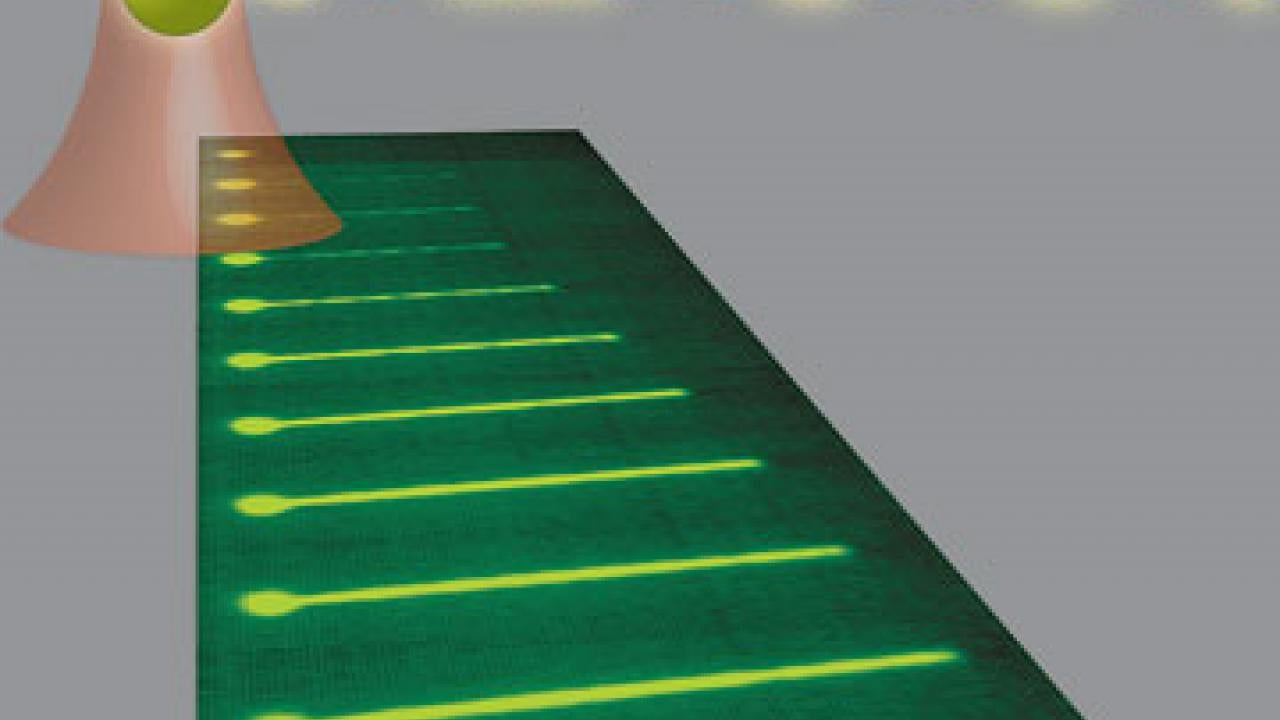
The DNA/Rad51 filament grows longer as more and more Rad51 molecules assemble onto it. The bright sphere on the left is the optical trap that holds the DNA.
Stephen Kowalczykowski/UC Davis video
Download Adobe Flash (free)
Human DNA is under constant assault from harmful agents such as ultraviolet sunlight, tobacco smoke and a myriad of chemicals, both natural and man-made. Because damage can lead to cancer, cell death and mutations, an army of proteins and enzymes are mobilized into action whenever it occurs.
Rad51 takes a leading role in the action. Always on call in the cell, molecules of the protein assemble into a long filament along a damaged or broken segment of DNA, where they help stretch out the coiled strands and align them with corresponding segments on the cell’s second copy of the chromosome, which serves as a template for reconstruction. Because this protein is regulated by a gene linked to increased risk of breast cancer, BRCA2, it is also thought to play a role in suppression of that disease.
With the ability to watch the assembly of individual filaments of Rad51 in real time, Kowalczykowski's team made a number of discoveries. Among those are that, in contrast to their bacterial counterparts, Rad51 filaments don’t grow indefinitely. This indicates that there is an as-yet undiscovered mechanism that regulates the protein’s growth, Kowalczykowski said.
Another surprising difference between the human and bacterial processes, Kowalczykowski said, is that Rad51 doesn’t fall away from the DNA when repair is complete. Instead, proteins that motor along DNA are required to dislodge it.
“From a practical point of view, being able to record these single molecules gives us insightful information regarding the assembly process,” the researcher said. “Now we’re able to measure this in a quantifiably meaningful way.”
Other contributors to the study were postdoctoral scholars Jovencio Hilario and Ichiro Amitani.
Media Resources
Liese Greensfelder, Research news (emphasis: biological and physical sciences, and engineering), (530) 752-6101, lgreensfelder@ucdavis.edu
Stephen Kowalczykowski, Microbiology, 530-752-5938, sckowalczykowski@ucdavis.edu