Researchers have long known that high levels of a specific protein in human cells are linked to tumor growth — but no one has fully understood how.
Now, a groundbreaking discovery by Kateryna Feoktistova, a graduate student, and Christopher Fraser, assistant professor, Department of Molecular and Cellular Biology, illuminates the way in which the protein, eukaryotic initiation factor 4E, or eIF4E, acts upon cancer-promoting messenger RNA molecules. When translated, this type of messenger RNA, or mRNA, can trigger the runaway cell replication that results in malignancies.
Published in the Aug. 13 edition of the Proceedings of the National Academy of Sciences, the results solve a decades-old scientific mystery and may lead to new, highly specific cancer treatments that will act only on growth-promoting cells as opposed to all cells.
“This protein is one the most important initiation factors in this cellular pathway, and there is a lot of energy in the cell that goes into regulating the level and availability of it,” Fraser said. “To suddenly find this function is quite a transformative idea in the field; people can now try and study this new activity and its relation to growth-promotion and cancer.”
With elevated eIF4E levels found in 30 percent of all major cancers, the protein is already a target of pharmaceutical research. Clinical trials are under way for drugs that act upon eIF4E’s long-known function of binding the cap at the head of all mRNA.
But the protein’s cap-binding activity does not fully explain its relationship to cancer.
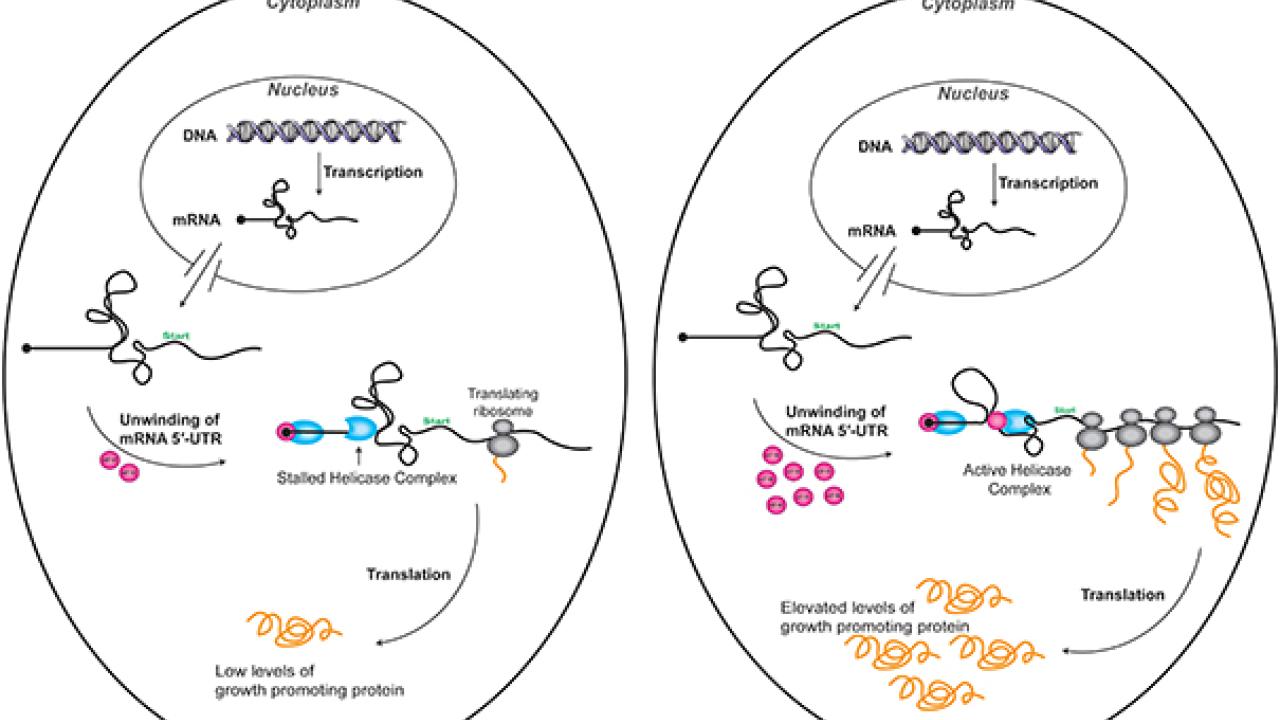
In research funded by the National Institutes of Health, Feoktistova and Fraser solved the mystery while studying a region at the head of mRNA strands, where many cancer-promoting mRNA are much more complex than typical mRNA. In the cancer-promoting variants, a highly knotted region at the start of the strand must be unwound before a ribosome can bind and begin translating the genetic code into the amino acids that build our cellular proteins.
Unwinding the knot
Usually that knotted region prevents most of the ribosomes from starting the process, so cancer-promoting mRNAs are not translated effectively. But when high levels of eIF4E are present, the 4E binds to a complex that activates another protein, 4A, which then is able to unwind the knot and translate the genetic code into proteins that can trigger tumor growth.
McGill University (Montreal) Professor Nahum Sonenberg, who discovered elF4E in 1978, expressed his enthusiasm for what he called “logical, beautiful” research.
“This is really a big discovery," he said. "It explains a lot of the biology that we have. At the beginning of my talks, I used to emphasize that mRNAs are eIF4E-sensitive, but people would ask, 'How does it work?’— and I never had an answer.
"In the last month, I have been able to mention Chris Fraser’s paper and finally provide an explanation.
“It has made my life much easier,” Sonenberg joked.
Feoktistova, who received her undergraduate degree at UC Davis and is now a fifth-year doctoral student, made the breakthrough when she purified the individual components of eIF4E’s larger protein complex and then observed the complex’s activity with individual parts missing. It was during this process that she first noticed 4E’s stimulation of 4A.
“Without 4E, the complex wasn’t active," she said. "People hadn’t studied it without 4E before because its individual protein components were very hard to separate."
Interaction between 4E and the complex containing 4A must be maintained for the unwinding process to finish, Fraser added.
“If you lose this connection, you stall the complex,” he said. “But if you have a lot of 4E floating around in the cell, and the complex loses a 4E, another one can be easily found.” See illustration.
Now researchers can add the stimulation of 4A and its unwinding activity to 4E’s long known — and totally separate — cap-binding activity.
Dean James E.K. Hildreth of the College of Biological Sciences said: “Textbooks are likely to be revised once the finding is confirmed, given the fundamental importance of this protein in cell physiology and the fact that essentially nothing new has been discovered regarding its function for several decades."
Fraser and Feoktistova’s next steps will be to study the ways that 4E, 4A and the rest of the complex work together to translate mRNA.
Towner is a senior writer in the College of Biological Sciences.
Media Resources
Dave Jones, Dateline, 530-752-6556, dljones@ucdavis.edu