Quick Summary
- “Game-changer for our ability to begin to fight the pandemic,” Dean Allison Brashear says
- 200 volunteers at UC Davis Health will be among some 30,000 participants worldwide
- Pharmaceutical giant Pfizer Inc. and smaller BioNTech of Germany lead the trial
Patients at UC Davis Health on Thursday (Aug. 20) became the first in Sacramento to receive a vaccine candidate for COVID-19, which is part of a major clinical trial involving 30,000 participants worldwide.
The six people who received the injections are part of a late-stage study that will enroll about 200 participants through UC Davis Health’s clinical trials program.
“I’m excited about being able to be afforded the opportunity to help the world tackle this pandemic,” said one of the first people vaccinated: Lucas Solano, 27, who enrolled in the trial a few days ago. “It’s exciting to be one of the people that essentially would take the initiative and be helpful, if this vaccine became approved and distributed worldwide.”
The trial headed by Pfizer Inc. and BioNTech is ongoing and still accepting participants. Learn about enrollment criteria and how to sign up.
The research trial, known as a phase 2/3 study, seeks to determine the efficacy and side effects of a single nucleoside-modified messenger RNA (modRNA) candidate from the pharmaceutical companies’ BNT162 mRNA-based vaccine program.
The vaccine candidate has undergone rigorous evaluation in the United States and Germany and has previously shown significant positive results, according to Pfizer.
UC Davis Health, which includes Sacramento’s only academic medical center, is one of only 120 sites taking part in the trial. The pharmaceutical giant Pfizer, which has teamed up with the smaller BioNTech of Germany, selected vaccine study sites known for their world-class research experience, infrastructure, and nearby concentrations of known and anticipated positive COVID-19 cases.
‘Historic day’
UC Davis has played an active role in seeking solutions to combat the coronavirus disease since the UC Davis Medical Center providers in February diagnosed and treated the first apparent case of COVID-19 acquired by community spread in the United States.
“This is a historic day for UC Davis School of Medicine,” Dean Allison Brashear said Thursday. “This is a game-changer for our ability to begin to fight the pandemic.”
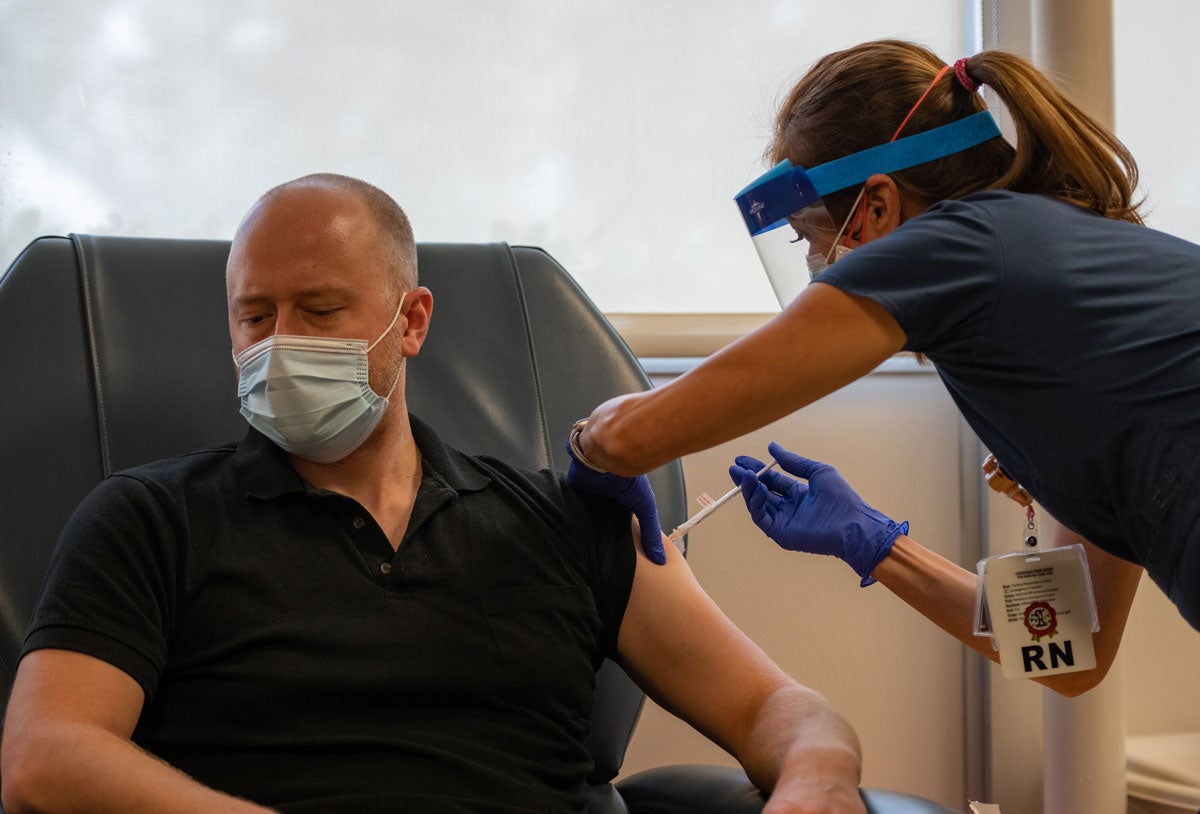
In the randomized trial, half the participants will receive the vaccine and half the placebo. Patients won’t know which they are getting and neither will most of the researchers. The study will measure safety, immune response and efficacy data needed for regulatory review.
The vaccine is one of several being developed around the world, at record-setting speed, in efforts to stop the spread of the virus which has killed more than 170,000 people in the United States, including about 12,000 in California.
Multiple departments involved
“To ramp up on a vaccination study like this, given the current climate with the virus, is extremely huge,” said Chris Kain, a nurse manager and the associate director of the Clinical Research Center within the Clinical and Translational Science Center. Kain coordinated with multiple departments before the first participants received their shots.
“It’s been amazing to see all the teams working together,” he said.
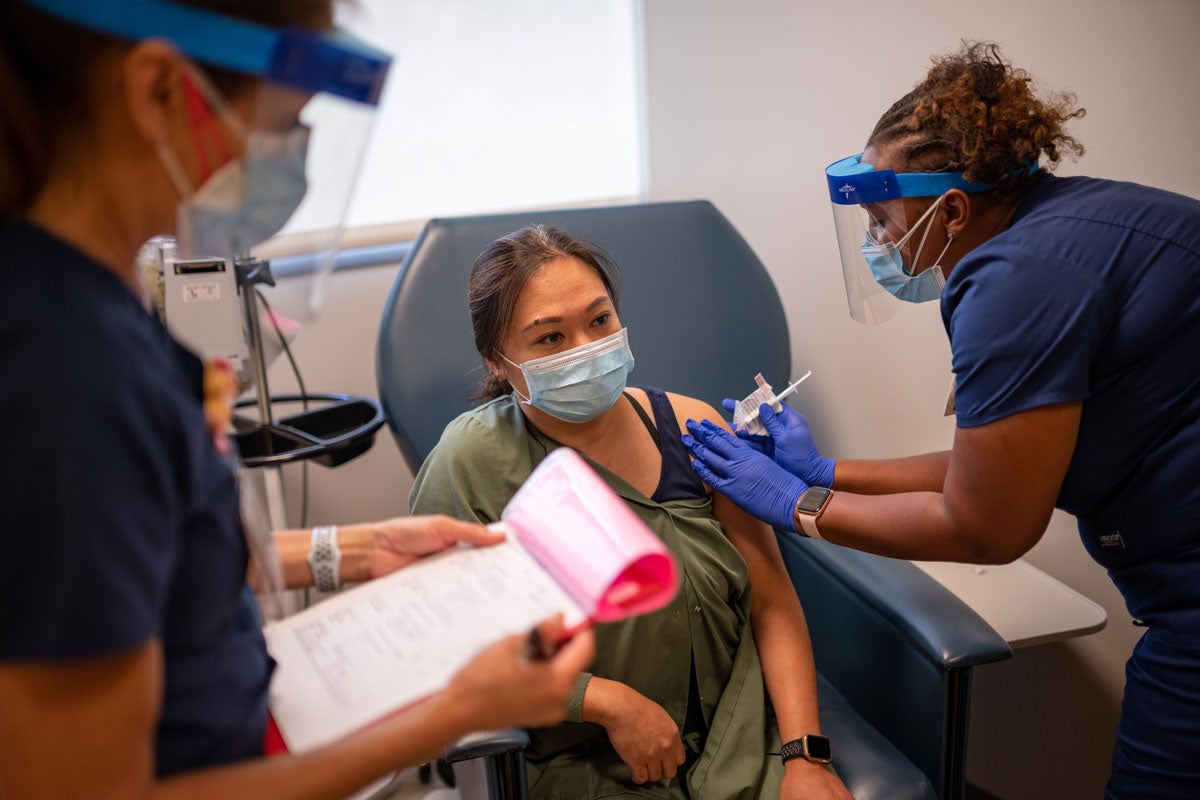
UC Davis Health serves a geographical region populated with multiple races and ethnicities, which increases the chances of identifying clinical trial candidates from more diverse backgrounds, compared to other communities in the United States. Latinos and Blacks have been disproportionately affected by the virus and are encouraged to enroll in the study. Health care workers and those who work in settings with a high volume of customers, such as grocery stores, are encouraged to participate.
The trial’s primary goal is preventing COVID-19 in those who have not been infected by SARS-CoV-2 prior to immunization and preventing COVID-19 regardless of whether participants have previously been infected by SARS-CoV-2. A secondary goal is preventing severe COVID-19 in those groups.
If the vaccine candidate’s success continues, Pfizer and its European partner BioNTech have stated they are on track to seek regulatory review as early as October. If regulatory authorization or approval is obtained, the companies plan to supply up to 100 million doses by the end of 2020 and approximately 1.3 billion doses by the end of 2021.
See earlier coverage: UC Davis Health to Enroll Participants for Major COVID-19 Vaccine Clinical Trial
Media Resources
UC Davis Health